SYMDEKO® RESULTS
Keep in mind, results shown are an average for all people studied and differed among individuals and mutations. You or your child may have a different experience.
What is SYMDEKO?
IMPORTANT SAFETY INFORMATION
Before taking SYMDEKO, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems
- are allergic to SYMDEKO or any ingredients in SYMDEKO. See the Patient Information for a list of ingredients
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if SYMDEKO will harm your unborn baby. You and your doctor should decide if you will take SYMDEKO while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if SYMDEKO passes into your breast milk. You and your doctor should decide if you will take SYMDEKO while you are breastfeeding
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What is SYMDEKO® (tezacaftor/ivacaftor and ivacaftor)?
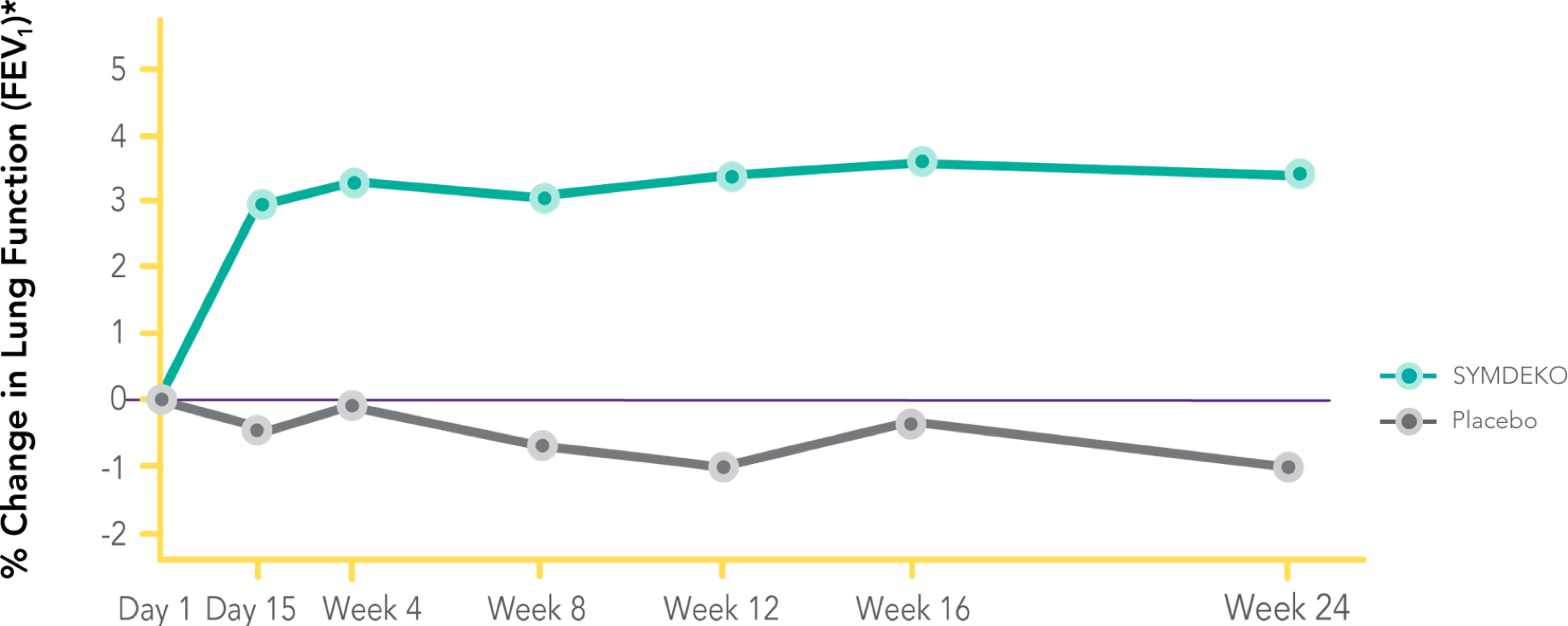
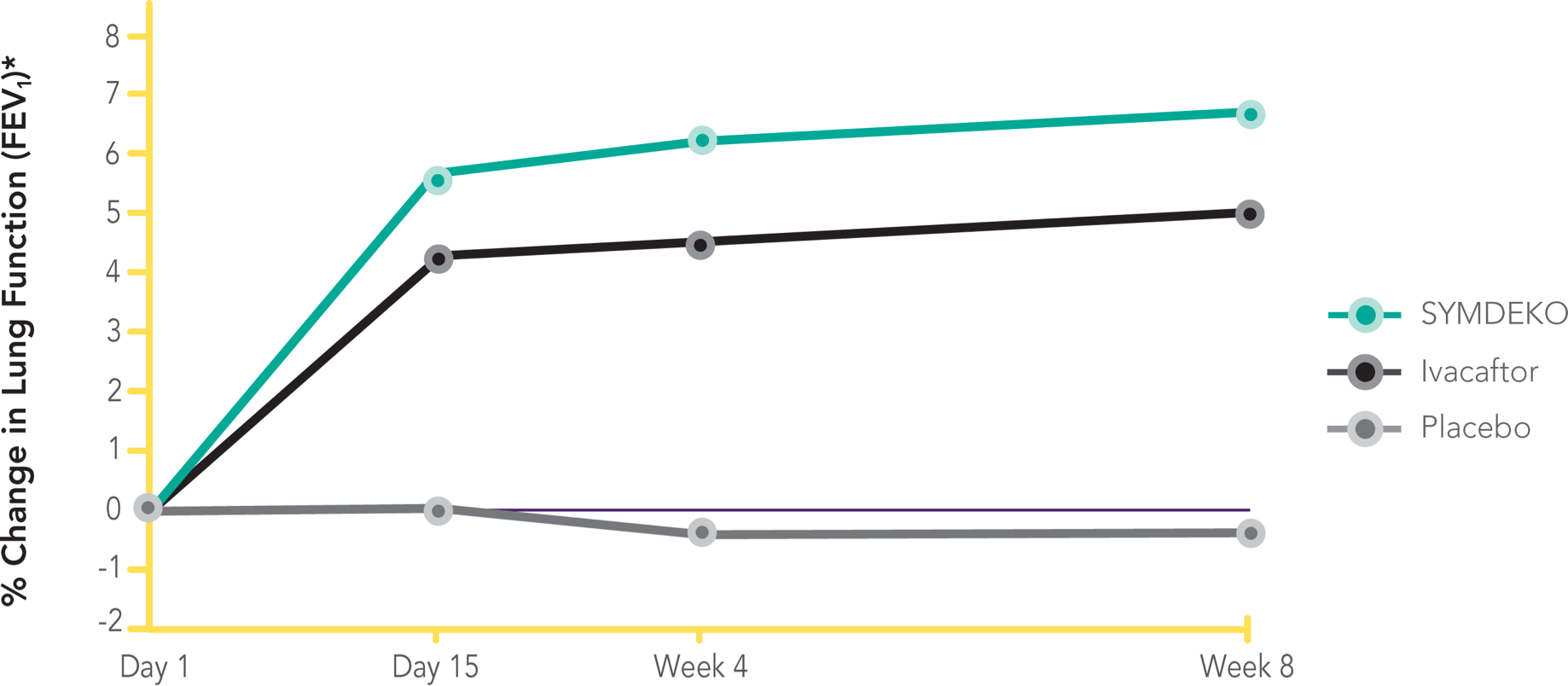
SYMDEKO is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have two copies of the F508del mutation, or who have at least one mutation in the CF gene that is responsive to treatment with SYMDEKO.
Talk to your doctor to learn if you have an indicated CF gene mutation.
It is not known if SYMDEKO is safe and effective in children under 6 years of age.
Important safety information
What is SYMDEKO® (tezacaftor/ivacaftor and ivacaftor)?
SYMDEKO is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have two copies of the F508del mutation, or who have at least one mutation in the CF gene that is responsive to treatment with SYMDEKO.
Talk to your doctor to learn if you have an indicated CF gene mutation.
It is not known if SYMDEKO is safe and effective in children under 6 years of age.
Before taking SYMDEKO, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems
- are allergic to SYMDEKO or any ingredients in SYMDEKO. See the Patient Information for a list of ingredients
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if SYMDEKO will harm your unborn baby. You and your doctor should decide if you will take SYMDEKO while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if SYMDEKO passes into your breast milk. You and your doctor should decide if you will take SYMDEKO while you are breastfeeding
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
SYMDEKO may affect the way other medicines work and other medicines may affect how SYMDEKO works. The dose of SYMDEKO may need to be adjusted when taken with certain medicines. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Especially tell your doctor if you take:
- antibiotics such as rifampin (RIFAMATE®, RIFATER®) or rifabutin (MYCOBUTIN®)
- seizure medicines such as phenobarbital, carbamazepine (TEGRETOL®, CARBATROL®, EQUETRO®), or phenytoin (DILANTIN®, PHENYTEK®)
- St. John’s wort
- antifungal medicines such as ketoconazole, itraconazole (such as SPORANOX®), posaconazole (such as NOXAFIL®), voriconazole (such as VFEND®), or fluconazole (such as DIFLUCAN®)
- antibiotics such as telithromycin, clarithromycin (such as BIAXIN®), or erythromycin (such as ERY-TAB®)
What should I avoid while taking SYMDEKO?
- SYMDEKO can cause dizziness in some people who take it. If you experience dizziness, do not drive or operate machines until symptoms improve.
- Avoid food or drink that contains grapefruit while you are taking SYMDEKO
What are the possible side effects of SYMDEKO?
SYMDEKO can cause serious side effects, including:
- High liver enzymes in the blood have been reported in people treated with SYMDEKO or treated with ivacaftor alone. Your doctor will do blood tests to check your liver:
- before you start SYMDEKO
- every 3 months during your first year of taking SYMDEKO
- every year while you are taking SYMDEKO
Your doctor may do blood tests to check the liver more often if you have had high liver enzymes in your blood in the past.
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- yellowing of your skin or the white part of your eyes
- loss of appetite
- nausea or vomiting
- dark, amber-colored urine
- Serious allergic reactions have happened to people who are treated with SYMDEKO. Call your doctor or go to the emergency room right away if you have any symptoms of an allergic reaction. Symptoms of an allergic reaction may include:
- rash or hives
- tightness of the chest or throat or difficulty breathing
- light-headedness or dizziness
- Abnormality of the eye lens (cataract) has happened in some children and adolescents treated with SYMDEKO or with ivacaftor alone. If you are a child or adolescent, your doctor should perform eye examinations before and during treatment with SYMDEKO to look for cataracts
The most common side effects of SYMDEKO include headache, nausea, sinus congestion, and dizziness.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of SYMDEKO. Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
For further information, please see full Prescribing Information, including Patient Information.
IMPORTANT SAFETY INFORMATION
Before taking SYMDEKO, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems
- are allergic to SYMDEKO or any ingredients in SYMDEKO. See the Patient Information for a list of ingredients
- have kidney problemsg
- are pregnant or plan to become pregnant. It is not known if SYMDEKO will harm your unborn baby. You and your doctor should decide if you will take SYMDEKO while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if SYMDEKO passes into your breast milk. You and your doctor should decide if you will take SYMDEKO while you are breastfeeding
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Before taking SYMDEKO, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if SYMDEKO will harm your unborn baby. You and your doctor should decide if you will take SYMDEKO while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if SYMDEKO passes into your breast milk. You and your doctor should decide if you will take SYMDEKO while you are breastfeeding
SYMDEKO may affect the way other medicines work, and other medicines may affect how SYMDEKO works.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Because the dose of SYMDEKO may need to be adjusted when taken with certain medicines.
Especially tell your doctor if you take:
- antifungal medicines such as ketoconazole (e.g., NIZORAL®), itraconazole (e.g., SPORANOX®), posaconazole (e.g., NOXAFIL®), voriconazole (e.g., VFEND®), or fluconazole (e.g., DIFLUCAN®)
- antibiotics such as telithromycin (e.g., KETEK®), clarithromycin (e.g., BIAXIN®), or erythromycin (e.g., ERY-TAB®)
What should I avoid while taking SYMDEKO?
- SYMDEKO can cause dizziness in some people who take it. Do not drive a car, use machinery, or do anything that needs you to be alert until you know how SYMDEKO affects you
- Avoid food or drink that contains grapefruit while you are taking SYMDEKO
What are the possible side effects of SYMDEKO?
SYMDEKO can cause serious side effects, including:
- High liver enzymes in the blood have been reported in people treated with SYMDEKO or treated with ivacaftor alone. Your doctor will do blood tests to check your liver:
- before you start SYMDEKO
- every 3 months during your first year of taking SYMDEKO
- every year while you are taking SYMDEKO
Your doctor may do blood tests to check the liver more often if you have had high liver enzymes in your blood in the past.
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- yellowing of your skin or the white part of your eyes
- loss of appetite
- nausea or vomiting
- dark, amber-colored urine
- Abnormality of the eye lens (cataract) in some children and adolescents treated with SYMDEKO or with ivacaftor alone. If you are a child or adolescent, your doctor should perform eye examinations before and during treatment with SYMDEKO to look for cataracts
The most common side effects of SYMDEKO include headache, nausea, sinus congestion, and dizziness.
These are not all the possible side effects of SYMDEKO. Call your doctor for medical advice about side effects. You are encouraged to report side effects to FDA at 1-800-FDA-1088.
For further information, please see full Prescribing Information, including Patient Information.
What is SYMDEKO?
IMPORTANT SAFETY INFORMATION
Before taking SYMDEKO, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems
- are allergic to SYMDEKO or any ingredients in SYMDEKO. See the Patient Information for a list of ingredients
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if SYMDEKO will harm your unborn baby. You and your doctor should decide if you will take SYMDEKO while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if SYMDEKO passes into your breast milk. You and your doctor should decide if you will take SYMDEKO while you are breastfeeding
What is SYMDEKO® (tezacaftor/ivacaftor and ivacaftor)?
SYMDEKO® (tezacaftor/ivacaftor and ivacaftor) is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have two copies of the F508del mutation, or who have at least one mutation in the CF gene that is responsive to treatment with SYMDEKO.
Talk to your doctor to learn if you have an indicated CF gene mutation.
It is not known if SYMDEKO is safe and effective in children under 6 years of age.
Important safety information
What is SYMDEKO® (tezacaftor/ivacaftor and ivacaftor)?
SYMDEKO is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have two copies of the F508del mutation, or who have at least one mutation in the CF gene that is responsive to treatment with SYMDEKO.
Talk to your doctor to learn if you have an indicated CF gene mutation.
It is not known if SYMDEKO is safe and effective in children under 6 years of age.
Before taking SYMDEKO, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if SYMDEKO will harm your unborn baby. You and your doctor should decide if you will take SYMDEKO while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if SYMDEKO passes into your breast milk. You and your doctor should decide if you will take SYMDEKO while you are breastfeeding
SYMDEKO may affect the way other medicines work, and other medicines may affect how SYMDEKO works.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your doctor if you take:
- antifungal medicines such as ketoconazole (e.g., NIZORAL®), itraconazole (e.g., SPORANOX®), posaconazole (e.g., NOXAFIL®), voriconazole (e.g., VFEND®), or fluconazole (e.g., DIFLUCAN®)
- antibiotics such as telithromycin (e.g., KETEK®), clarithromycin (e.g., BIAXIN®), or erythromycin (e.g., ERY-TAB®)
What should I avoid while taking SYMDEKO?
- SYMDEKO can cause dizziness in some people who take it. Do not drive a car, use machinery, or do anything that needs you to be alert until you know how SYMDEKO affects you
- Avoid food or drink that contains grapefruit while you are taking SYMDEKO
What are the possible side effects of SYMDEKO?
SYMDEKO can cause serious side effects, including:
- High liver enzymes in the blood have been reported in people treated with SYMDEKO or treated with ivacaftor alone. Your doctor will do blood tests to check your liver:
- before you start SYMDEKO
- every 3 months during your first year of taking SYMDEKO
- every year while you are taking SYMDEKO
Your doctor may do blood tests to check the liver more often if you have had high liver enzymes in your blood in the past.
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- yellowing of your skin or the white part of your eyes
- loss of appetite
- nausea or vomiting
- dark, amber-colored urine
- Abnormality of the eye lens (cataract) in some children and adolescents treated with SYMDEKO or with ivacaftor alone. If you are a child or adolescent, your doctor should perform eye examinations before and during treatment with SYMDEKO to look for cataracts
The most common side effects of SYMDEKO include headache, nausea, sinus congestion, and dizziness.
These are not all the possible side effects of SYMDEKO. Call your doctor for medical advice about side effects. You are encouraged to report side effects to FDA at 1-800-FDA-1088.
For further information, please see full Prescribing Information, including Patient Information.
Important Safety Information
Before taking SYMDEKO, tell your doctor about all of your medical conditions, including if you:
- have or have had liver problems
- are allergic to SYMDEKO or any ingredients in SYMDEKO. See the Patient Information for a list of ingredients
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if SYMDEKO will harm your unborn baby. You and your doctor should decide if you will take SYMDEKO while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if SYMDEKO passes into your breast milk. You and your doctor should decide if you will take SYMDEKO while you are breastfeeding
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
SYMDEKO may affect the way other medicines work and other medicines may affect how SYMDEKO works. The dose of SYMDEKO may need to be adjusted when taken with certain medicines. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Especially tell your doctor if you take:
- antibiotics such as rifampin (RIFAMATE®, RIFATER®) or rifabutin (MYCOBUTIN®)
- seizure medicines such as phenobarbital, carbamazepine (TEGRETOL®, CARBATROL®, EQUETRO®), or phenytoin (DILANTIN®, PHENYTEK®)
- St. John’s wort
- antifungal medicines such as ketoconazole, itraconazole (such as SPORANOX®), posaconazole (such as NOXAFIL®), voriconazole (such as VFEND®), or fluconazole (such as DIFLUCAN®)
- antibiotics such as telithromycin, clarithromycin (such as BIAXIN®), or erythromycin (such as ERY-TAB®)
What should I avoid while taking SYMDEKO?
- SYMDEKO can cause dizziness in some people who take it. If you experience dizziness, do not drive or operate machines until symptoms improve.
- Avoid food or drink that contains grapefruit while you are taking SYMDEKO
What are the possible side effects of SYMDEKO?
SYMDEKO can cause serious side effects, including:
- High liver enzymes in the blood have been reported in people treated with SYMDEKO or treated with ivacaftor alone. Your doctor will do blood tests to check your liver:
- before you start SYMDEKO
- every 3 months during your first year of taking SYMDEKO
- every year while you are taking SYMDEKO
Your doctor may do blood tests to check the liver more often if you have had high liver enzymes in your blood in the past.
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- yellowing of your skin or the white part of your eyes
- loss of appetite
- nausea or vomiting
- dark, amber-colored urine
- Serious allergic reactions have happened to people who are treated with SYMDEKO. Call your doctor or go to the emergency room right away if you have any symptoms of an allergic reaction. Symptoms of an allergic reaction may include:
- rash or hives
- tightness of the chest or throat or difficulty breathing
- light-headedness or dizziness
- Abnormality of the eye lens (cataract) has happened in some children and adolescents treated with SYMDEKO or with ivacaftor alone. If you are a child or adolescent, your doctor should perform eye examinations before and during treatment with SYMDEKO to look for cataracts
The most common side effects of SYMDEKO include headache, nausea, sinus congestion, and dizziness.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of SYMDEKO. Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
For further information, please see full Prescribing Information, including Patient Information.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
SYMDEKO may affect the way other medicines work and other medicines may affect how SYMDEKO works. The dose of SYMDEKO may need to be adjusted when taken with certain medicines. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Especially tell your doctor if you take:
- antibiotics such as rifampin (RIFAMATE®, RIFATER®) or rifabutin (MYCOBUTIN®)
- seizure medicines such as phenobarbital, carbamazepine (TEGRETOL®, CARBATROL®, EQUETRO®), or phenytoin (DILANTIN®, PHENYTEK®)
- St. John’s wort
- antifungal medicines such as ketoconazole, itraconazole (such as SPORANOX®), posaconazole (such as NOXAFIL®), voriconazole (such as VFEND®), or fluconazole (such as DIFLUCAN®)
- antibiotics such as telithromycin, clarithromycin (such as BIAXIN®), or erythromycin (such as ERY-TAB®)
What should I avoid while taking SYMDEKO?
- SYMDEKO can cause dizziness in some people who take it. If you experience dizziness, do not drive or operate machines until symptoms improve.
- Avoid food or drink that contains grapefruit while you are taking SYMDEKO
What are the possible side effects of SYMDEKO?
SYMDEKO can cause serious side effects, including:
- High liver enzymes in the blood have been reported in people treated with SYMDEKO or treated with ivacaftor alone. Your doctor will do blood tests to check your liver:
- before you start SYMDEKO
- every 3 months during your first year of taking SYMDEKO
- every year while you are taking SYMDEKO
Your doctor may do blood tests to check the liver more often if you have had high liver enzymes in your blood in the past.
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- yellowing of your skin or the white part of your eyes
- loss of appetite
- nausea or vomiting
- dark, amber-colored urine
- Serious allergic reactions have happened to people who are treated with SYMDEKO. Call your doctor or go to the emergency room right away if you have any symptoms of an allergic reaction. Symptoms of an allergic reaction may include:
- rash or hives
- tightness of the chest or throat or difficulty breathing
- light-headedness or dizziness
- Abnormality of the eye lens (cataract) has happened in some children and adolescents treated with SYMDEKO or with ivacaftor alone. If you are a child or adolescent, your doctor should perform eye examinations before and during treatment with SYMDEKO to look for cataracts
The most common side effects of SYMDEKO include headache, nausea, sinus congestion, and dizziness.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of SYMDEKO. Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.
For further information, please see full Prescribing Information, including Patient Information.